Mediterranean BioMedical Journals
International Journal of Medicine and Surgery
Volume 1, Issue 2, 2014
META-ANALYSIS
Dr Bouti Khalidª, MD, Dr Borki Rajaeᵇ, MD, Dr Fenane Hichamᶜ, MD, Pr Laila Herrakᵈ, MD.
ª Center of Tuberculosis and Lung Diseases, Tetouan, Morocco.
ᵇ Department of Otolaryngology, Head and Neck Surgery, Specialties Hospital, Rabat, Morocco.
ᶜ Department of Thoracic Surgery, El Farabi Hospital, Oujda, Morocco.
ᵈ Department of pulmonology, Ibn Sina Hospital, Rabat, Morocco.
Received October 19, 2014; Revised November 07, 2014; Accepted November 21, 2014.
ABSTRACT
Background: Cannabis is the illicit psychoactive substance the most consumed in the world. Little is known about the association between the use of cannabis and the risk of lung cancer.
Objective:The aim of this meta-analysis is to determine whether use of cannabis is a risk factor for lung cancer.
Methods: We conducted a systematic review and meta-analyses of all languages articles using relevant computerised databases. MEDLINE (online PubMed), Web of knowledge, Embase, EBSCO CINAHL, ScienceDirect, Scopus, Cochrane Library, and Directory of Open Access Journals were searched to September 2014 for cohorts and case-control studies that assessed the risk of lung cancer associated with cannabis smoking. The literature search was performed with a combination of medical subject headings terms, "cannabis" and "lung neoplasms". Data extraction: Two investigators independently analysed and extracted results from eligible studies.
Our study's registration number on PROSPERO is CRD42014008872.
Results: The search strategy identified 2476 citations. 13 studies were eligible for inclusion: 2 pooled analysis of 9 case-control studies, one case-control study and 3 cohorts.
The cumulative analysis for all the studies under a fixed-effects model showed that cannabis smoking determined an increased risk of developing lung cancer in the future (relative risk 1.22, 95% confidence interval 0.999–1.5; p=0.05), with no evidence of heterogeneity across the studies (I2: 34%; p¼0.01).
Conclusions: The use of cannabis with or without tobacco smoking is associated with an increased risk for lung cancer.
KEYWORDS
Cannabis, lung cancer, risk factor, positive association, systematic review, meta-analysis.
Corresponding author:
Dr Khalid Bouti: Center of Tuberculosis and Lung Diseases, Tetouan, Morocco.
Email: khalid.bouti@um5s.net.ma
Copyright © 2014 Khalid Bouti et al.
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cannabis is the illicit psychoactive substance the most consumed in the world (1). It is a generic term for preparations (e.g. marijuana, hashish and hash oil) derived from the Cannabis sativa plant. The three main forms of cannabis products are the flower or herb (marijuana), resin (hashish), and oil (hashish oil), and their relative levels of consumption vary globally by region. Resin dominates the markets in the Near- and Middle-East as well as South-west Asia; resin and herb markets are comparable in size in Northern Africa and Europe; whereas cannabis herb dominates the rest of the world including North America (World Drug Report 2012).
Marijuana and tobacco smoke contain many of the same potent carcinogens (2, 3) , but with marijuana smoke condensates being even more cytotoxic and mutagenic (2-5) . In comparison with tobacco smoking, marijuana smoking is usually associated with deeper inhalation, longer breath-holding times, use of unfiltered marijuana cigarettes (‘‘joints’’), and relatively greater delivery of tar (a carcinogenic particulate matter) to the lungs (3, 6) . Cannabis cigarettes are less densely packed than tobacco cigarettes, and tend to be smoked without filters (7, 8) .
Tobacco smoking is the major cause of lung cancer (3, 9) . Links between smoking and lung cancer are clearly established since the work of Doll and Peto (10-12) . This is helpful as long as a large percentage of the high-risk population can be easily identified via a smoking history. But, little is known about the association between the use of cannabis and the risk of lung cancer (13-15) . Epidemiological evidence for an association between cannabis and lung cancer is limited and conflicting. Case series have suggested a causative role for cannabis in lung cancer in young adults (7, 16, 17) . The case–control studies published to date have shown both the presence
(7, 13, 15, 18) and absence (7, 14) of an association, but have been limited by the inability to quantify use (7, 13, 15, 18) , confounding with combined cannabis and tobacco use, and studies being undertaken in populations in which use may have serious legal consequences resulting in potential information bias and poor response rates (7, 13-15, 18) . The weakenss of some studies was due to small sample sizes, dichotomous classification of marijuana use (which might obscure threshold effects), selection and recall biases, and lack of adjustment for tobacco use in statistical modeling (3, 6) .
Several studies have demonstrated pre-cancerous histological and molecular abnormalities in the respiratory tracts of cannabis smokers, and the carcinogenic effects of cannabis smoke have been demonstrated in vitro and in different in vivo animal models (7, 19-25) .
Furthermore, experimental studies support an association between marijuana smoke exposure and
lung cancer, with lung cancer cell lines demonstrating tetrahydrocannabinol (THC) induced malignant cell proliferation and a murine model suggesting that THC promotes tumor growth by inhibiting antitumor immunity by a cannibinoid-2 receptor mediated pathway (6, 24, 26, 27) .
The objective of this meta-analysis is to determine whether use of cannabis is a risk factor for lung cancer.
METHODS
A protocol was developed and systematic methods were used to identify relevant studies, assess study eligibility for inclusion, and evaluate study quality. The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2009) guidelines (28). The meta-analysis conforms to the guidelines outlined by the Meta-analysis of Observational Studies in Epidemiology recommendations (29).
Inclusion and Exclusion Criteria
This systematic review and meta-analysis incorporated retrospective and prospective cohort, cross-sectional, and case-control studies meeting the following inclusion criteria: 1/ the study reported original, empirical research published in a peer-reviewed journal, 2/ the study adults with current or former cannabis consumption as study population, and 3/ adults who never smoked cannabis as controls. Studies reporting data on adolescents (under 18 years of age) were excluded. Included studies reported odds ratios (ORs), risk ratios (RRs), and confidence intervals (CIs) comparing those who smoked used to those who never smoked cannabis.
Search Strategy
Eight electronic databases: MEDLINE (online PubMed), Web of knowledge, Embase, EBSCO CINAHL, ScienceDirect, Scopus, Cochrane Library, and Directory of Open Access Journals up to 31 Septembre 2014, were searched using full text and Medical Subject Headings (MeSH) terms to identify studies reporting an association between lung cancer and cannabis smoking. Truncation of terms was used to capture variation in terminology. The search was not restricted to the English language, nor restricted by any other means. Searches were conducted using synonyms and combinations of the following search terms: Cannabi, Hemp Plant, Cannabis indica, Marihuana, Marijuana, Marijuanas, Ganja, Hashish, Hemp, Bhang, Cannabis sativa in one hand, and Pulmonary Neoplasms, Lung Neoplasm, Lung Cancer, Pulmonary Cancer in the other hand. Additional studies were identified by reviewing the reference lists of all included studies and by using a forward citation search to identify more recent studies that had cited included studies. EndNote X7 software was used to manage the references.
Data Collection and Quality Assessment
Studies obtained from the database searches were independently assessed by two researchers (KB and RB) in three phases: title, abstract, and full paper screening. Any disagreements at any of the screening stages were resolved by discussion between the two researchers in the first instance and with two reviewers (HF and LH) if agreement could not be reached. The coders were not masked to the journals or authors of the studies reviewed. Data extraction was carried out by KB using an Access database pro-forma developed for this purpose, and double-checked by RB. A standardised data extraction sheet was developed, and data retrieved included publication details, country where study was conducted, methodological characteristics such as sample size and study design, smoking, cannabis smoking, and health outcomes. The data extraction sheet included a quality assessment tool to rate the methodological quality of each study based on the Newcastle-Ottawa Scale for assessing the quality of observational studies (30). Two authors was contacted for further information but they didn’t reply.
Statistical Analyses
All analyses were performed using comprehensive meta-analysis (Comprehensive Meta-Analysis, Version 2.2.064, 8 November 2011; Biostat, 14 North Dean Street, Englewood, NJ).
We pooled results from the individual studies. We used fixed and random effect models to calculate summary relative risks (RRs) and 95% confidence intervals (CIs). The natural logarithm of the RRs was estimated and the RR from each study by the inverse of its variance was weighted. A two tailed p<0.05 was considered statistically significant. Statistical heterogeneity was evaluated using the I2 statistic, which assesses the appropriateness of pooling the individual study results. The I2 value provides an estimate of the amount of variance across studies due to the heterogeneity rather than chance.
RESULTS
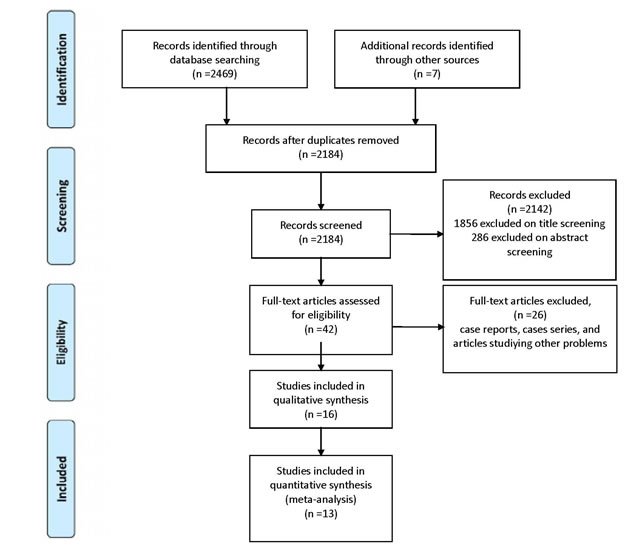
A flow diagram of the search results is presented in the PRISMA flow diagram (Figure 1). A total of 2184 records were identified through electronic databases. Removing search overlap and irrelevant studies based on title and abstract review reduced the results to 42 records. After full-text analysis, 16 studies were chosen for inclusion in qualitative synthesis, and finally 13 studies met the criteria for meta-analysis (two pooled analysis, three cohorts, and nine case-control studies). Two of the cohorts were prospective, and one was retrospective. None of the studies contained individual data.
Figure 1: PRISMA flow diagram

Study characteristics, including design, populations, year of publication, and countries where studies were done, and outcomes are presented in Figure 2.
Figure 2: Characteristics of studies includes in the Meta-analysis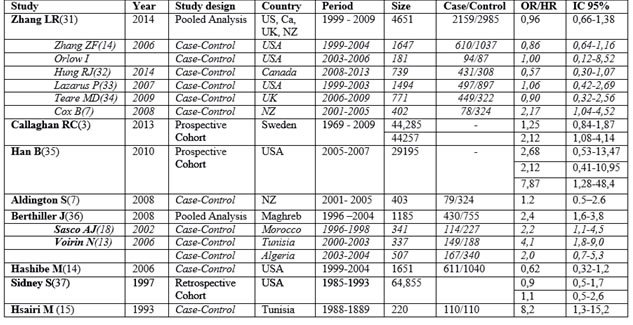
Hsairi et al.(15) conducted in Tunisia between 1988 and 1889 a Case-Control study (n=220) with 110 cases and 110 controls. He found a positive association of cannabis consumption and lung cancers with adjusted odds ratio (OR) of 8,2 (confidence interval (CI) 95 % : 1,3—15,2), compared to never users of cannabis.
Sidney et al.(37) studied 64,855 examinees in the United States between 1979 and 1985 with self-administered questionnaires about smoking habits, including marijuana use. Follow-up for cancer incidence was conducted through 1993 (mean length 8.6 years). Compared with nonusers/experimenters (lifetime use of less than seven times), ever- and current use of marijuana were not associated with increased risk of lung cancer with OR=0,9 (IC 95 % : 0,5—1,7) in men, and OR=1,1 (IC 95 % : 0,5—2,6) in women.
Hashibe et al. (14) studied between 1999 and 2004 a sample of 1651 with 611 lung cancer cases and
1,040 cancer-free controls and found the adjusted OR estimate (and IC 95 %) for >60 versus 0 joint-years was 0.62 (0.32, 1.2) for lung cancer. No association was consistently monotonic across exposure categories, and restriction to subjects who never smoked cigarettes yielded similar findings.
Berthiller et al. published a pooled analysis of three hospital-based case-control studies conducted in Morocco (18, 36) , Tunisia (13, 36) , and Algeria (36) . The protocols of the three studies were similar.
The Moroccan study was a hospital based case-control study included 118 cases and 235controls that were enrolled in Casablanca, between January 1996 and January 1998. Women from this study were excluded from the pooled analysis because of the small number (four cases and eight controls). Combined use of hashish/kiff and snuff had an OR of 6.67 (1.65-26.90), whereas the OR for hashish/kiff (without snuff) was 1.93 (0.57-6.58). Ever cannabis exposure compared to never cannabis exposure had an odds ratio of 2.2 (95% CI: 1,1-4,5).(18) .
The Tunisian study was a hospital based case-control study conducted among men only between March 2000 and February 2003 and included 149 cases and 188 controls. The odds ratio for the past use of cannabis and lung cancer was 4.1 (95% CI: 1.9-9.0) after adjustment for age, tobacco use, and occupational exposures (13) .
The Algerian study was a hospital based case-control study conducted in the Wilaya of Setif, Algeria between March 2003 and December 2004 and included 167 cases and 340 controls. The odds ratio for the past use of cannabis and lung cancer was 2.0 (95% CI: 0.7-5.3) after adjustment (36) .
The pooled analysis results showed ninety-six percent of cases and 67.8% of controls were tobacco smokers and 15.3% of cases and 5% of controls were ever cannabis smokers. All cannabis smokers were tobacco users. Adjusting for country, age, tobacco smoking, and occupational exposure, the odds ratio (OR) for lung cancer was 2.4 (95% CI: 1.6 –3.8) for ever cannabis smoking. The OR among current tobacco users and ever cannabis smokers was 18.2 (95% CI: 8.0–41.0). The risk of lung cancer increased with increasing joint-years, but not with increasing dose or duration of cannabis smoking (36) .
Aldington et al. (7), studied in New Zealand between January 2001 and July 2005, 79 cases of lung cancer and 324 controls. The relative risk of lung cancer for cannabis users after adjustment for age, sex, ethnicity, family history of lung cancer and pack-yrs of cigarette smoking was 1.2 (95% CI 0.5–2.6). The study concluded also that long-term cannabis use increases the risk of lung cancer in young adults.
Han et al. (35) analyzed data from respondents aged 35 to 49 from the 2005–2007 National Surveys on Drug Use and Health (NSDUH) and found after adjustment for potential confounding factors, the results of multivariate logistic regression models indicated positive associations between duration of marijuana use and lung cancer with odds ratio of 2.68 (95% CI 0.53–13.47) for light users (≤1 year), 2.12 (95% CI 0.41–10.95) for moderate users (2-10 years), and 7.87 (95% CI 1.28–48.40) for heavy smokers (≥ 11 years).
Callaghan et al. (3) examined in Sweden a population-based cohort study examined men (n = 49,321) aged 18–20 years old assessed for cannabis use and other relevant variables during military conscription in Sweden in 1969–1970. Participants were tracked until 2009 for incident lung cancer outcomes in nationwide linked medical registries. The cohort concluded that heavy (lifetime use of more than 50 times) cannabis smoking was significantly associated to lung cancer. The adjusted hazard ratios (HRs) and 95 % CIs for lung cancer (n = 179) among 44,257 conscripts, in relation to lifetime frequency of cannabis-use categories was 2.12, (95 % CI 1.08–4.14). The adjusted hazard ratios (HRs) and 95 % CIs for lung cancer (n = 179) among 44,285 conscripts in relation to lifetime history of cannabis-use categories (‘‘ever vs. never’’) was 1.25 (0.84–1.87).
Zhang et al. (31) investigated the association between cannabis smoking and lung cancer risk, and analysed data on 2,159 lung cancer cases and 2,985 controls were pooled from 6 case-control studies in the US, Canada, UK, and New Zealand within the International Lung Cancer Consortium. With adjustment for sociodemographic factors, tobacco smoking status and pack-years, the overall pooled OR for habitual versus nonhabitual or never users was 0.96 (95% CI: 0.66–1.38). Compared to nonhabitual or never users, the summary OR was 0.88 (95% CI: 0.63–1.24) for individuals who smoked 1 or more joint-equivalents of cannabis per day and 0.94 (95%CI: 0.67–1.32) for those consumed at least 10 joint-years.
Figure 3: Meta-analysis
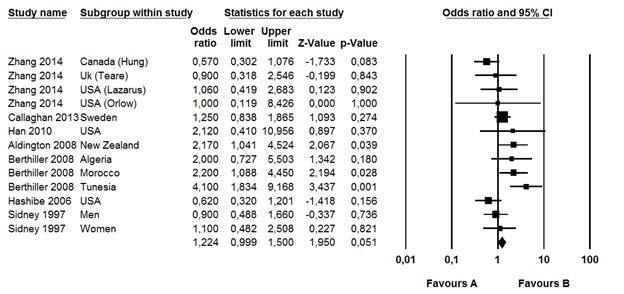
The study of Hsairi (15) was excluded from the meta-analysis because log values for lower and upper limits were not symmetric.
The cumulative analysis for all the studies under a fixed-effects model (Figure 3) showed that cannabis smoking determined an increased risk (þ45%) of developing lung cancer in the future (relative risk 1.22, 95% confidence interval 0.999–1.5; p=0.05), with no evidence of heterogeneity across the studies (I2: 34%; p¼0.01).
DISCUSSION
Exposure of cell cultures to cannabis smoking or cannabinoid induces DNA damages, human lung cell division dysfunction, a reduction of apoptosis pulmonary cells of human cancers, and lung cell proliferation cancer. Bronchial mucosa exposure to cannabis smoke induces the same histological lesions that induces tobacco smoke.
Some studies suggested that cannabinoids may have some antitumor activities on formed tumors (38, 39) . Cannabis smoke consists of the same components as tobacco smoke, however the inhalation way is different witch is more irritating to the bronchi and make them more vulnerable to carcinogens (40).
Experimental studies have demonstrated that exposure to cannabis smoke and/or principal cannabinoids induces cell damage prior to tumor transformation (27, 39, 41, 42) .
The dose of cannabis product consumption differs by source of plant material, its processing, and by smoking techniques including the depth of inhalation and breath-holding, number and frequency of puffs, as well as how much of the joint is smoked (31). Cannabis is usually smoked without a filter, and smoking dynamics studies among habitual marijuana users show that the overall burden of particulates delivered to the respiratory tract is about 4 times greater when smoking marijuana than when smoking the same amount of tobacco (31, 43) .
Hsairi et al.(15, 36) conducted a hospital based case-control study in Tunisia where the OR of bronchial cancer for cannabis smokers compared with nonusers, adjusted for age, sex, cigarettes per day, snuff tobacco and water pipe use was 8.2 (95% CI:1.3–15.5).
The results of Berthiller (36) study witch is a pooled analysis of three hospital based case-control studies conducted in Morocco, Tunisia, and Algeria supported a positive association between cannabis smoking and lung cancer. A 2.4-fold increase in the risk of lung cancer among men was estimated for ever cannabis smokers compared with never users after adjustment for age, tobacco smoking, occupational exposures, and country. The country profiles of Morocco, Tunisia, and Algeria are similar with regard to their cultural and historical use of cannabis (36, 44) , as well as their tobacco use. The prevalence of cannabis smoking in 2000 was 7.4% in Morocco according to the United Nations Office on Drugs and Crime (UNODC) report (36, 44) and was 8% among the controls from Morocco. Data on cannabis smoking in Algeria and Tunisia were not available from the UNODC report. The prevalence of tobacco smoking among men in 2000 was 34.5% in Morocco, 46% in Tunisia, and 43.8% in Algeria according to the World Health Organization Surveillance of Risk Factors Report and the Tobacco Control Country Profiles.(36, 45) . All three studies were hospital based and a potential selection bias, particularly for controls, cannot be excluded. Another potential bias may arise from the absence of histologic confirmation for 43 cases out of 430. 10% (43 of 430) were radiologically diagnosed without histologic confirmation, and most of these cases were from Morocco (41 of 43).
In a study that compared tar, carbon monoxide, and pH levels in smoke from marijuana and tobacco cigarettes, higher pH and tar levels were found in marijuana cigarettes than in tobacco cigarettes (8). Further, cannabis cigarettes are usually composed of a mixture of tobacco and cannabis, and the strong effects of cannabis consumption might in part be explained by exposure to the high levels of tar that are usually found in Tunisian tobacco as well as possible deeper inhalation of the smoke among cannabis users (8). In contrast, two large cohort studies in California did not find a positive association between marijuana smoking and lung cancer (14, 36, 37) .
In Aldington et al. (7) population-based, case–control study, we find evidence of a relationship between smoking cannabis and lung cancer in young adults. For each joint-yr of cannabis exposure the risk of lung cancer was estimated to increase by 8%. A population-based control group was used rather than a hospital-based control group as the latter is susceptible to significant bias due to the many medical conditions associated with cannabis use (46, 47).
A major differential risk between cannabis and cigarette smoking was observed, with one joint of cannabis being similar to 20 cigarettes for risk of lung cancer. This is consistent with the observation that smoking ‘‘a few’’ cannabis joints a day causes similar histological changes in the tracheobronchial epithelium as smoking 20–30 tobacco cigarettes a day (38) .
In this regard, cannabis smoke has been shown to have greater concentrations of the carcinogenic polyaromatic hydrocarbons benz[a]pyrene and benz[a]anthracene than cigarette smoke (25).
Callaghan et al.(3) found in his population-based cohort study of young Swedish males aged 18–20 years old at conscription (1969–1970), that heavy cannabis smoking, defined as self-reported lifetime use of at least 50 times, was significantly associated with more than a twofold risk HR 2.12 (95 % CI 1.08–4.14) of developing lung cancer over the 40-year follow-up period, even after statistical adjustment for baseline tobacco use and other potential confounders. In addition, as demonstrated in a large body of work (9) , a strong dose–response relation in this cohort was observed between baseline self-reported tobacco smoking and lung cancer outcomes.
Marijuana smoking is highly prevalent in many countries around the world, especially among youth (48, 49).Our results show that the use of cannabis with or without tobacco smoking is associated with an increased risk for lung cancer.
These findings do raise concern about the potential lung cancer risk associated with marijuana use ever in life especially given the possibility that such marijuana smoking may occur during a ‘‘critical period’’ of lung cancer susceptibility to carcinogens in marijuana smoke (50). Among youth, cannabis is often perceived as the least harmful of illicit drugs (51),
Restricted follow-up stands as a substantial limitation, especially given that most lung cancer cases appear much later in life (e.g., the recent median age of first lung cancer diagnosis is approximately 70 years of age) (52, 53). In particular, cohort studies need to include large numbers of cannabis smokers (who are found most commonly in late adolescence and early adulthood), with a full range of light-to-heavy marijuana use, and incorporate an extended follow-up period into late adulthood (when lung cancer becomes clinically manifest).
While it is important to interpret our findings in the context of limitations, the balance of evidence would suggest a positive association between cannabis and lung cancer.
CONCLUION
The use of cannabis with or without tobacco smoking is associated with an increased risk for lung cancer. This issue is of major public health importance, due to the prevalent use of cannabis globally and lung cancer being responsible for over a million deaths in the world each year (54) .
Our Results also can contribute to the current ongoing debate about the legalization and decriminalization of cannabis in many countries (55).
This study is a start, but more studies with significant findings, which control for confounding variables, are required for clarity of whether cannabis use as a risk factor for lung cancer.
SOURCE OF SUPPORT
Declared none.
COMPETING INTERESTS
The authors declare no competing interests.
AUTHORS’ CONTRIBUTIONS
The participation of each author corresponds to the criteria of authorship and contributorship emphasized in the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals of the International Committee of Medical Journal Editors:
Conceived and designed the experiments: KB RB. Performed the experiments: KB RB HF LH. Analyzed the data: KB RB. Wrote the first draft of the manuscript: KB. Contributed to the writing of the manuscript: RB HF LH. Criteria for authorship read and met: KB RB HF LH. Agree with manuscript results and conclusions: KB RB HF LH.
ACKNOWLEDGEMENT
Declared none.
REFERENCES
[1] Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-91. ![]()
![]()
[2] Maertens RM, White PA, Rickert W, Levasseur G, Douglas GR, Bellier PV, et al. The genotoxicity of mainstream and sidestream marijuana and tobacco smoke condensates. Chemical research in toxicology. 2009;22(8):1406-14. ![]()
![]()
[3] Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer causes & control : CCC. 2013;24(10):1811-20.![]()
![]()
[4] Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chemical research in toxicology. 2008;21(2):494-502.![]()
![]()
[5] Marselos M, Karamanakos P. Mutagenicity, developmental toxicity and carcinogenicity of cannabis. Addiction biology. 1999;4(1):5-12.![]()
![]()
[6] Mehra R, Moore BA, Crothers K, Tetrault J, Fiellin DA. The association between marijuana smoking and lung cancer: a systematic review. Archives of internal medicine. 2006;166(13):1359-67.![]()
![]()
[7] Aldington S, Harwood M, Cox B, Weatherall M, Beckert L, Hansell A, et al. Cannabis use and risk of lung cancer: a case–control study. European Respiratory Journal. 2008;31(2):280-6.![]()
![]()
[8] Rickert WS, Robinson JC, Rogers B. A comparison of tar, carbon monoxide and pH levels in smoke from marihuana and tobacco cigarettes. Canadian journal of public health = Revue canadienne de sante publique. 1982;73(6):386-91.![]()
[9] Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):29s-55s.![]()
![]()
[10] Underner M, Urban T, Perriot J, de Chazeron I, Meurice JC. [Cannabis smoking and lung cancer]. Revue des maladies respiratoires. 2014;31(6):488-98.![]()
![]()
[11] Doll R, Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. British medical journal. 1976;2(6051):1525-36.![]()
![]()
[12] Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. Journal of epidemiology and community health. 1978;32(4):303-13.![]()
![]()
[13] Voirin N, Berthiller J, Benhaim-Luzon V, Boniol M, Straif K, Ayoub WB, et al. Risk of lung cancer and past use of cannabis in Tunisia. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2006;1(6):577-9.![]()
![]()
[14] Hashibe M, Morgenstern H, Cui Y, Tashkin DP, Zhang ZF, Cozen W, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer epidemiology, biomarkers & prevention. 2006;15(10):1829-34.![]()
![]()
[15] Hsairi M, Achour N, Zouari B, Ben Romdhane H, Achour A, Maalej M, et al. [Etiologic factors in primary bronchial carcinoma in Tunisia]. La Tunisie medicale. 1993;71(5):265-8.![]()
[16] Sridhar KS, Raub WA, Jr., Weatherby NL, Metsch LR, Surratt HL, Inciardi JA, et al. Possible role of marijuana smoking as a carcinogen in the development of lung cancer at a young age. Journal of psychoactive drugs. 1994;26(3):285-8.![]()
![]()
[17] Fung M, Gallagher C, Machtay M. Lung and aero-digestive cancers in young marijuana smokers. Tumori. 1999;85(2):140-2.![]()
[18] Sasco AJ, Merrill RM, Dari I, Benhaim-Luzon V, Carriot F, Cann CI, et al. A case-control study of lung cancer in Casablanca, Morocco. Cancer causes & control : CCC. 2002;13(7):609-16.![]()
![]()
[19] Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112(2):319-26.![]()
![]()
[20] Gong H, Jr., Fligiel S, Tashkin DP, Barbers RG. Tracheobronchial changes in habitual, heavy smokers of marijuana with and without tobacco. The American review of respiratory disease. 1987;136(1):142-9.![]()
![]()
[21] Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. Journal of the National Cancer Institute. 1998;90(16):1198-205.![]()
![]()
[22] Busch FW, Seid DA, Wei ET. Mutagenic activity of marihuana smoke condensates. Cancer letters. 1979;6(6):319-24.![]()
![]()
[23] Roy PE, Magnan-Lapointe F, Huy ND, Boutet M. Chronic inhalation of marijuana and tobacco in dogs: pulmonary pathology. Research communications in chemical pathology and pharmacology. 1976;14(2):305-17.![]()
[24] Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, et al. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. Journal of immunology (Baltimore, Md : 1950). 2000;165(1):373-80.![]()
![]()
[25] Hoffmann D, Brunnemann K, Gori G, Wynder E. On the carcinogenicity of marijuana smoke. Recent Advances in Phytochemistry: Springer; 1975. p. 63-81.![]()
[26] Galve-Roperh I, Sanchez C, Cortes ML, Gomez del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nature medicine. 2000;6(3):313-9.![]()
![]()
[27] Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer research. 2004;64(6):1943-50.![]()
![]()
[28] Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264-9.![]()
![]()
[29] Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama. 2000;283(15):2008-12.![]()
![]()
[30] Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
[31] Zhang LR, Morgenstern H, Greenland S, Chang SC, Lazarus P, Teare MD, et al. Cannabis smoking and lung cancer risk: Pooled analysis in the International Lung Cancer Consortium. International journal of cancer Journal international du cancer. 2014.![]()
![]()
[32] Wang Y, McKay JD, Rafnar T, Wang Z, Timofeeva MN, Broderick P, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. 2014;46(7):736-41.![]()
![]()
[33] Gallagher CJ, Muscat JE, Hicks AN, Zheng Y, Dyer A-M, Chase GA, et al. The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiology Biomarkers & Prevention. 2007;16(4):823-8.![]()
![]()
[34] ReSoLuCENT PJW. Resource for the Study of Lung Cancer Epidemiology in North Trent.vol. 2013, 2009, 45.
[35] Han B, Gfroerer JC, Colliver JD. Associations Between Duration of Illicit Drug Use and Health Conditions: Results from the 2005–2007 National Surveys on Drug Use and Health. Annals of Epidemiology.20(4):289-97.![]()
![]()
[36] Berthiller J, Straif K, Boniol M, Voirin N, Benhaim-Luzon V, Ayoub WB, et al. Cannabis smoking and risk of lung cancer in men: a pooled analysis of three studies in Maghreb. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3(12):1398-403.![]()
![]()
[37] Sidney S, Quesenberry CP, Jr., Friedman GD, Tekawa IS. Marijuana use and cancer incidence (California, United States). Cancer causes & control : CCC. 1997;8(5):722-8.![]()
[38] Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. American journal of respiratory and critical care medicine. 1998;157(3 Pt 1):928-37.![]()
![]()
[39] Leuchtenberger C, Leuchtenberger R. The effects of naturally occurring metabolites (L-cysteine, vitamin C) on cultured human cells exposed to smoke of tobacco or marijuana cigarettes. Cytometry. 1984;5(4):396-402.![]()
![]()
[40] Underner M, Urban T, Perriot J, Peiffer G, Meurice JC. [Cannabis use and impairment of respiratory function]. Revue des maladies respiratoires. 2013;30(4):272-85.![]()
![]()
[41] Chiesara E, Cutrufello R, Rizzi R. Chromosome Damage in Heroin-Marijuana and Marijuana Addicts. In: Chambers C, Chambers P, Gitter S, editors. Toxicology in the Use, Misuse, and Abuse of Food, Drugs, and Chemicals. Archives of Toxicology. 6: Springer Berlin Heidelberg; 1983. p. 128-30.![]()
![]()
[42] Murison G, Chubb CB, Maeda S, Gemmell MA, Huberman E. Cannabinoids induce incomplete maturation of cultured human leukemia cells. Proceedings of the National Academy of Sciences. 1987;84(15):5414-8.![]()
![]()
[43] Wu T-C, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. New England Journal of Medicine. 1988;318(6):347-51.![]()
![]()
[44] Drugs UNOo. World drug report 2010: United Nations Publications; 2010.
[45] Shafey O, Dolwick S, Guindon GE. Tobacco control country profiles. Atlanta: American Cancer Society. 2003;356.
[46] Khalsa JH, Genser S, Francis H, Martin B. Clinical consequences of marijuana. The Journal of Clinical Pharmacology. 2002;42(S1):7S-10S.![]()
![]()
[47] Hall W, Solowij N. Adverse effects of cannabis. The Lancet. 1998;352(9140):1611-6.![]()
![]()
[48] Hibell B, Guttormsson U, Ahlström S, Balakireva O, Bjarnason T, Kokkevi A, et al. The 2011 ESPAD Report. Substance Use Among Students in 36 European Countries. Tukholma: The Swedish Council for Information on Alcohol and other Drugs, 2012. Viitattu 27.9. 2013.
[49] Degenhardt L, Bucello C, Calabria B, Nelson P, Roberts A, Hall W, et al. What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug and alcohol dependence. 2011;117(2):85-101.![]()
![]()
[50] Wiencke JK, Kelsey KT. Teen smoking, field cancerization, and a" critical period" hypothesis for lung cancer susceptibility. Environmental health perspectives. 2002;110(6):555.![]()
![]()
[51] Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975-2010. Volume I, Secondary School Students. Institute for Social Research. 2011.![]()
[52] Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. The Oncologist. 2007;12(1):20-37.![]()
![]()
[53] Holmberg L, Sandin F, Bray F, Richards M, Spicer J, Lambe M, et al. National comparisons of lung cancer survival in England, Norway and Sweden 2001–2004: differences occur early in follow-up. Thorax. 2010;65(5):436-41.![]()
![]()
[54] Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127(12):2893-917.![]()
![]()
[55] Room R. Cannabis policy: moving beyond stalemate: Oxford University Press; 2010.